Quick Facts
Back to All Types of MPS >
What is MPS I (Hurler, Hurler-Scheie, Scheie Syndrome)?
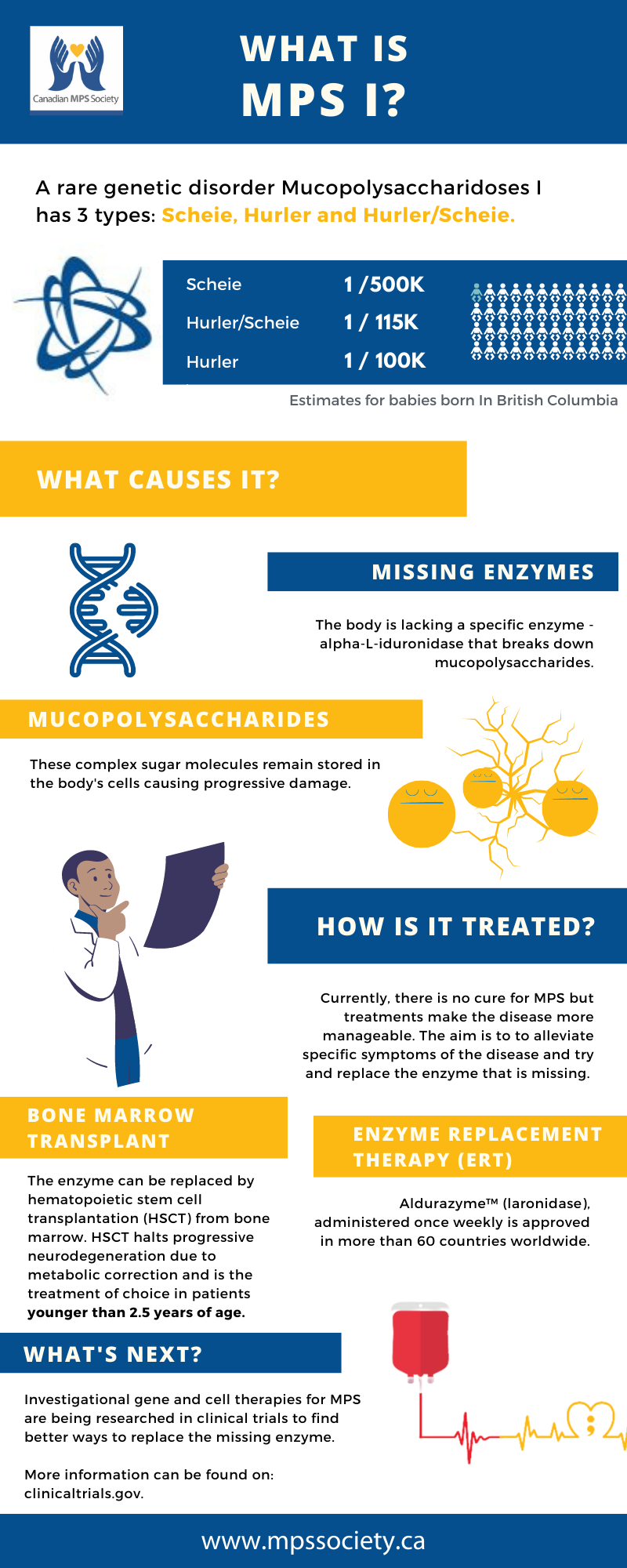
MPS I has also been called Hurler, Hurler-Scheie and Scheie syndrome depending on the severity of symptoms presented across a spectrum. The names are taken after the doctors that identified them. Hurler takes its name from Gertrude Hurler, the doctor who first described a boy and girl with the condition in 1919.
Those who are mildly affected are said to have Scheie syndrome. Individuals who seem not to fit clearly in either the severe or the mild end of the disease were said to have Hurler/Scheie.
There is no cure for MPS diseases, but there are ways of managing and treating the problems they cause.
What causes this disease?
Mucopolysaccharides are long chains of sugar molecule used in the building of connective tissues in the body.
- “saccharide” is a general term for a sugar molecule (think of saccharin).
- “poly” means many
- “muco” refers to the thick jelly-like consistency of the molecules
There is a continuous process in the body of replacing used materials and breaking them down for disposal. Children with these diseases are missing an enzyme called alpha-L-iduronidase which is essential in breaking down the mucopolysaccharides called dermatan sulfate and heparan sulfate. The incompletely broken down mucopolysaccharides remain stored in cells in the body causing progressive damage. Babies may show little sign of the disease, but as more and more cells become damaged, symptoms start to appear.
How common are these diseases?
It has been estimated that in British Columbia, 1 in 100,000 babies born would have Hurler. The estimate for Scheie is 1 in 500,000, births and for Hurler/Scheie it is 1 in 115,000. There is an estimate in the United States that 1 in 25,000 births will result in some form of MPS.
How is the disease inherited?
We all have genes inherited from our parents which control whether we are tall, short, fair, etc. Some genes we inherit are “recessive,” that is to say we carry the gene, but it does not have any affect on our development. MPS I (Hurler-Scheie syndrome ) is caused by a recessive gene.
If an adult carrying the abnormal gene marries another carrier, there will be a one in four chance with every pregnancy that the child will inherit the defective gene from each parent and will be affected with the disease.
There is a two in three chance that unaffected brothers and sisters of children with MPS I will be carriers. They can be reassured; however, that, as the disease is so rare, the chance of marrying another carrier is very slight provided they do not marry a cousin or other close family member.
Is there a cure for MPS I
There is no cure but treatments such as bone marrow transplantation and/or enzyme replacement therapy (ERT) can help make MPS I a more manageable disease. Aldurazyme is the first and only approved ERT treatment developed through recombinant DNA technology for individuals with MPS I. For more information, visit the treatment website at www.aldurazyme.com/patients
What are the treatments for MPS I?
Bone marrow transplantation and/or enzyme replacement therapy (ERT) can help make MPS I a more manageable disease.
Aldurazyme™ (laronidase), administered once weekly, is approved in more than 60 countries worldwide, and was licensed for use by Health Canada on May 31, 2004 for long-term enzyme replacement therapy in patients with a confirmed diagnosis of MPS I, to treat the non-neurological manifestations of the disease.
Aldurazyme was developed by BioMarin and SanofiGenzyme under a joint venture agreement. BioMarin manufactures the treatment and SanofiGenzyme handles the global sales and marketing.
For more information, speak to your physician or visit www.aldurazyme.com or contact SanofiGenzyme at 800-745-4447